BY LINDA BAKER
Researchers in a multitude of disciplines are searching for ways to soak up excess carbon dioxide, the compound that contributes to global warming.
BY LINDA BAKER
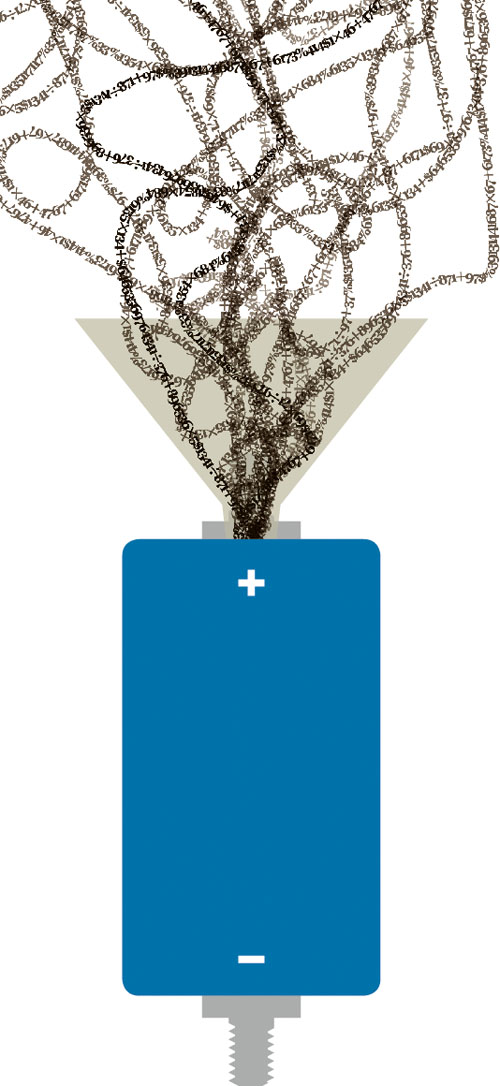 Researchers in a multitude of disciplines are searching for ways to soak up excess carbon dioxide, the compound that contributes to global warming. Now chemists at Oregon State University have found a way of using atmospheric CO2 to advance energy-storage technology. In a novel experiment, researchers heated a mix of magnesium and zinc metals amid a swirl of compressed carbon dioxide. The reaction produced “nanoporous graphene,” a material used in battery-like devices that store energy and release it rapidly. There are other ways of making graphene, says OSU chemistry professor Xiulei (David) Ji. But old-school methods involve expensive and toxic materials — a challenge for commercial applications. “The real innovation we brought in is scalability, which is important for industry,” Ji says. The CO2-based reaction also yields a stronger, higher-density material with greater conductivity. The takeaway? The nanomaterial will help companies create more powerful “supercapacitors,” high octane devices that are used in a wide range of consumer electronics and industry products, and hold special promise for improving the efficiency of hybrid vehicles and alternative energy storage. Will the process really make a dent in global warming? “By using CO2 to get this material, we cannot say we are solving climate change,” Ji observes. “But we are using a wasteful climate-change compound to produce a valuable material.”
Researchers in a multitude of disciplines are searching for ways to soak up excess carbon dioxide, the compound that contributes to global warming. Now chemists at Oregon State University have found a way of using atmospheric CO2 to advance energy-storage technology. In a novel experiment, researchers heated a mix of magnesium and zinc metals amid a swirl of compressed carbon dioxide. The reaction produced “nanoporous graphene,” a material used in battery-like devices that store energy and release it rapidly. There are other ways of making graphene, says OSU chemistry professor Xiulei (David) Ji. But old-school methods involve expensive and toxic materials — a challenge for commercial applications. “The real innovation we brought in is scalability, which is important for industry,” Ji says. The CO2-based reaction also yields a stronger, higher-density material with greater conductivity. The takeaway? The nanomaterial will help companies create more powerful “supercapacitors,” high octane devices that are used in a wide range of consumer electronics and industry products, and hold special promise for improving the efficiency of hybrid vehicles and alternative energy storage. Will the process really make a dent in global warming? “By using CO2 to get this material, we cannot say we are solving climate change,” Ji observes. “But we are using a wasteful climate-change compound to produce a valuable material.”


